A new therapy for children with curved spines was approved for general use after tests only on pigs and dead bodies, an investigation has revealed.
Magec rods are supposed to help straighten the spine.
But experts say the rods appear to snap too easily and say they are “very surprised” at how little evidence was needed to get them approved.
The manufacturers Nuvasive said there was always risk in treating scoliosis and Magec had rebuilt families’ lives.
BBC Panorama has been working with the International Consortium of Investigative Journalists and 58 media organisations around the world including The Guardian newspaper and the British Medical Journal.
A back like a shark
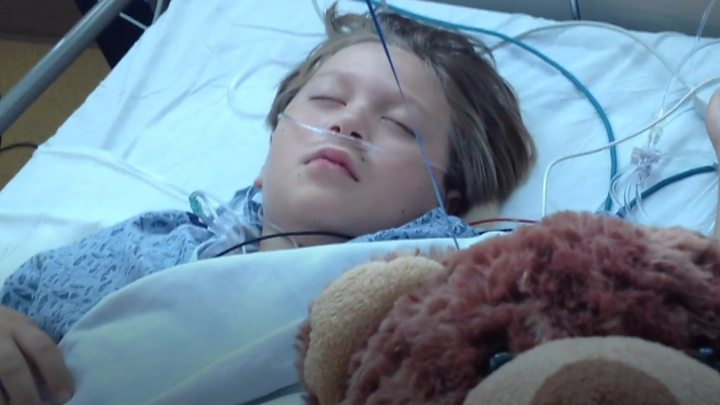
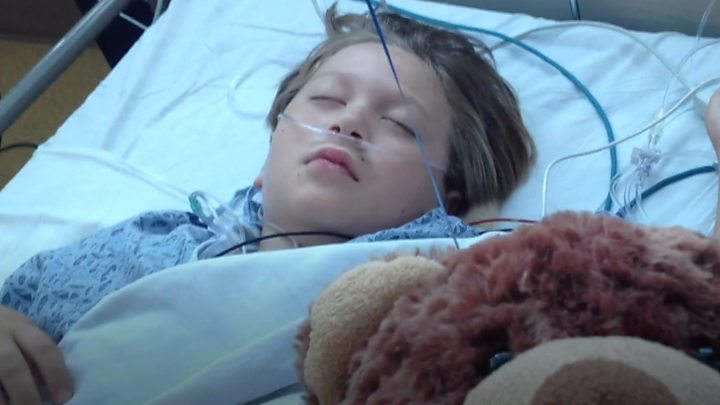
Anthony Wainess, from California in the US, had a severely twisted and curved spine from the disease scoliosis.
“All the time I bend down it’s like a shark’s fin at the back,” he said back when he was nine and before having treatment.
The standard treatment would have involved surgery to insert metal rods around the spine to straighten it.
But the rods need to be lengthened as children grow, which means operations every six months.
Image copyright
Ellipse and Mike Gibson
Magec rods and magnetic remote adjuster (L), from a promotional video, and an X-ray of a curved spine then partially straightened by the rods
Anthony was given Magec rods in 2013.
They are marketed as a safer and cheaper alternative because they can be lengthened with magnets while still in the body.
Anthony’s dad Steven did not want his son to have to face repeat operations to lengthen the rods in his spine and Magec rods seemed ideal.
But a year after they were fitted, one of the rods snapped inside him.
The rods were replaced, but a year later they snapped again and had to be permanently removed.
Steven said: “It was a very low point for me. And it made me wonder just how good was it to have these Magec rods in to begin with? Did we make a mistake?”
Europe first
The inventors got them approved for use across Europe via Germany – five years before the US – after studies on pigs and dead bodies.
In the UK, the rods were recommended by NICE, the body that decides best practice for the National Health Service, in 2014 based on “limited” evidence from a “small” studies in patients.
But before long, surgeons in the north-east of England began to raise concerns.
Mike Gibson, a consultant spinal surgeon at the Royal Victoria Infirmary in Newcastle and a former president of the British Scoliosis Society, has put the rods into 10 children.
But he does not think they work properly.
“When NICE turned around and said that it should be your implant of choice, I was frankly staggered,” he said.
Initially he gave rods he removed to the manufacturer, Nuvasive, to analyse, but heard nothing back.
So he sent rods to engineers at Newcastle University, led by Prof Tom Joyce, who found titanium metal debris had been leaking into children’s spines.
The rods were analysed by engineers at Newcastle University
Prof Joyce says nobody knows how the titanium might affect the children: “There’s a big question mark there. What are the long term effects of this?”
The Newcastle team has analysed 100 rods. Around 80 of them did not work as they should including problems lengthening.
Mr Gibson also thinks the rods become more vulnerable to breaking as they extend.
He questions why they were ever approved: “It’s very surprising they needed so little data,” he says.
The UK’s Medicines and Healthcare products Regulatory Agency (MHRA) said it could not comment on the safety and effectiveness of specific devices because of issues around confidentiality.
Graeme Tunbridge, a senior figure at the regulator, said: “It’s important to remember that before any implant is put into anyone, there’s a decision making process and there’s a discussion between a clinician and a patient to understand the risks involved in that.”
NICE says the decision to recommend Magec was taken after careful consideration of the clinical and economic evidence and that the company has not highlighted any safety issues to them.
The manufacturer Nuvasive says Magec rods have been shown to be effective and that they avoid the pain and complications of the regular operations required by conventional treatment.
“We see every day the challenges brought on by early-onset scoliosis and, while no technology in these patients is without risk, we have also seen Magec rebuild patient and family lives.”



